Featured Posts
Microneedling, also known as collagen induction therapy (CIT), is a science-backed treatment that stimulates your skin’s natural healing process to reveal a more radiant and refined complexion. When it is done right by a medical professional with the right training, tools, and serums, it is transformative.

Understanding Hormone Therapy & Menopause Treatment
HORMONE THERAPY know your hormone healthcare options As you enter midlife, understanding your health care
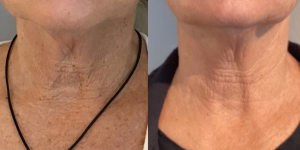
What is Radiesse?
Radiesse is a one-of-a-kind injectable treatment that offers dual benefits: immediate volume plus long-term collagen stimulation. Find out if it’s right for you.
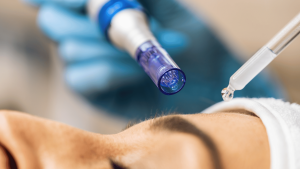
What is Collagen Induction Therapy?
Collagen Induction Therapy (CIT or microneedling) is a minimally invasive treatment that rejuvenates skin by stimulating collagen production. Learn more about this effective option for improving fine lines, scars and skin texture.
Everything You Need to Know About Medical Facials
Our medical facial is not a spa facial. It is a complete detail for your face and customized to your needs. Learn more about our most popular treatment.
Face
What is VISIA Imaging?
VISIA is a digital imaging system that evaluates your skin health not just on the surface, but across sub-surface levels as well.
What is Morpheus8?
Seeking out cutting-edge treatments and technology is a core value at Melinda Menezes, MD. We’re excited to announce the future of skin remodeling, collagen induction and anti-aging is available at MMMD! Learn more
Everything You Need to Know About Medical Facials
Our medical facial is not a spa facial. It is a complete detail for your face and customized to your needs. Learn more about our most popular treatment.
What is Collagen Induction Therapy?
Collagen Induction Therapy (CIT or microneedling) is a minimally invasive treatment that rejuvenates skin by stimulating collagen production. Learn more about this effective option for improving fine lines, scars and skin texture.
Body
What is Morpheus8?
Seeking out cutting-edge treatments and technology is a core value at Melinda Menezes, MD. We’re excited to announce the future of skin remodeling, collagen induction and anti-aging is available at MMMD! Learn more
skin
What is VISIA Imaging?
VISIA is a digital imaging system that evaluates your skin health not just on the surface, but across sub-surface levels as well.
What is Morpheus8?
Seeking out cutting-edge treatments and technology is a core value at Melinda Menezes, MD. We’re excited to announce the future of skin remodeling, collagen induction and anti-aging is available at MMMD! Learn more
What is Collagen Induction Therapy?
Collagen Induction Therapy (CIT or microneedling) is a minimally invasive treatment that rejuvenates skin by stimulating collagen production. Learn more about this effective option for improving fine lines, scars and skin texture.
What is Radiesse?
Radiesse is a one-of-a-kind injectable treatment that offers dual benefits: immediate volume plus long-term collagen stimulation. Find out if it’s right for you.
Best Self

What is my Kibbe Body Type and Personal Color Palette
Like everything we do at Melinda Menezes, MD, we want your clothes and accessories to help you feel like your best self. That’s why we’re now offering Kibbe Body Typing and Personal Color Analysis!

Understanding Hormone Therapy & Menopause Treatment
HORMONE THERAPY know your hormone healthcare options As you enter midlife, understanding your health care

Auntie Meenal’s Favorite Niece’s Chai Recipe
A reflection on life, love, and family recipes

Spiritual RX: Aura Hygiene Part III – Quickly Shift Your Vibe
Cranky, irritated, irritable, pissed, pooped. I could go on and on. There is a gang of feelings that are not enjoyable. And I don’t want to waste a bunch of time stuck in the yuck. Over the years, I’ve worked with friends, my therapist, and my Spiritual path to develop a way to shift out of the ick. I will share some techniques you can use and customize for your system/person.